What Is Lantus? (insulin glargine)
What is Lantus?
Lantus is the brand name of insulin glargine, a long-acting insulin used to treat adults and children with type 1 diabetes mellitus and adults with type 2 diabetes mellitusto control high blood sugar.
Lantus replaces the insulin that your body no longer produces. Insulin is a natural substance that allows your body to convert dietary sugar into energy and helps store energy for later use. In type 2 diabetes mellitus, your body does not produce enough insulin, or the insulin produced is not used properly, causing a rise in blood sugar. Like other types of insulin, Lantus is used to normalize blood sugar levels.
Controlling high blood sugar helps prevent kidney damage, blindness, nerve problems, loss of limbs, and sexual dysfunction. Proper control of diabetes has also been shown to reduce your risk of a heart attack or stroke.
Lantus is meant to be used alongside a proper diet and exercise program recommended by your doctor.
Lantus is manufactured by Sanofi-Aventis. It was approved for use by the Food and Drug Administration (FDA) in 2000 as the first long-acting human insulin administered once a day with a 24-hour sugar-lowering effect.
Lantus (insulin glargine) is a man-made form of a hormone that is produced in the body. Insulin is a hormone that works by lowering levels of glucose (sugar) in the blood. Insulin glargine is a long-acting insulin that starts to work several hours after injection and keeps working evenly for 24 hours.
Lantus is used to improve blood sugar control in adults and children with diabetes mellitus. Lantus is used to treat type 1 or type 2 diabetes in adults, and type 1 diabetes children who are at least 6 years old.
LANTUS (insulin glargine injection) is a sterile solution of insulin glargine for subcutaneous use. Insulin glargine is a recombinant human insulin analog that is a long-acting, parenteral blood-glucose-lowering agent. Insulin glargine has low aqueous solubility at neutral pH. At pH 4 insulin glargine is completely soluble. After injection into the subcutaneous tissue, the acidic solution is neutralized, leading to formation of microprecipitates from which small amounts of insulin glargine are slowly released, resulting in a relatively constant concentration/time profile over 24 hours with no pronounced peak. This profile allows oncedaily dosing as a basal insulin. LANTUS is produced by recombinant DNA technology utilizing a non-pathogenic laboratory strain of Escherichia coli (K12) as the production organism. Insulin glargine differs from human insulin in that the amino acidasparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the B-chain. Chemically, insulin glargine is 21A-Gly-30Ba-L-Arg-3030b-L-Arg-human insulin and has the empirical formula C267H404N72O78S6 and a molecular weight of 6063. Insulin glargine has the following structural formula:
LANTUS consists of insulin glargine dissolved in a clear aqueous fluid. Each milliliter of LANTUS (insulin glargine injection) contains 100 Units (3.6378 mg) insulin glargine.
The 10 mL vial presentation contains the following inactive ingredients per mL: 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, 20 mcg polysorbate 20, and water for injection.
The 3 mL prefilled pen presentation contains the following inactive ingredients per mL: 30 mcg zinc, 2.7 mg m-cresol, 20 mg glycerol 85%, and water for injection.
The pH is adjusted by addition of aqueous solutions of hydrochloric acid and sodium hydroxide. LANTUS has a pH of approximately 4
Important information
You should not use Lantus if you are having an episode of hypoglycemia (low blood sugar), or if you are in a state of diabetic ketoacidosis.
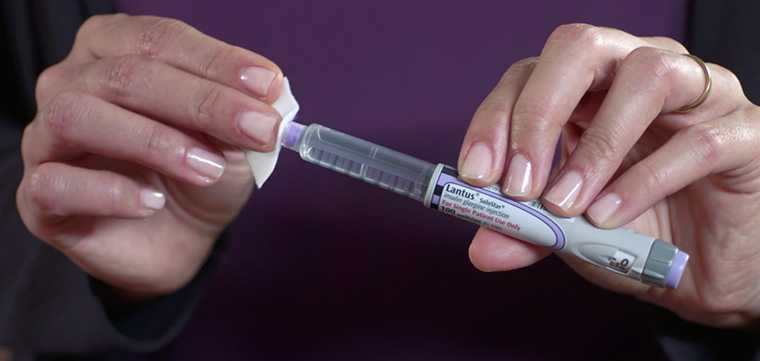
Never share a Lantus injection pen or cartridge with another person. Sharing injection pens or cartridges can allow disease such as hepatitis or HIV to pass from one person to another. Lantus is only part of a complete program of treatment that may also include diet, exercise, weight control, foot care, eye care, dental care, and testing your blood sugar. Follow your diet, medication, and exercise routines very closely. Changing any of these factors can affect your blood sugar levels.
Before taking this medicine
You should not use Lantus if you are allergic to insulin, or if you are having an episode of hypoglycemia (low blood sugar).
Lantus is not approved for use by anyone younger than 6 years old, and should not be used to treat type 2 diabetes in a child of any age.
To make sure Lantus is safe for you, tell your doctor if you have:
- liver or kidney disease;
- low levels of potassium in your blood (hypokalemia); or
- diabetic ketoacidosis (call your doctor for treatment).
Tell your doctor if you also take pioglitazone or rosiglitazone (sometimes contained in combinations with glimepiride or metformin). Taking certain oral diabetes medicines while you are using insulin may increase your risk of serious heart problems.
Follow your doctor’s instructions about using Lantus if you are pregnant or breast-feeding a baby. Blood sugar control is very important during pregnancy, and your dose needs may be different during each trimester of pregnancy. Your dose needs may also be different while you are breast-feeding.