Accutane Embryopathy (Fetal Retinoid Syndrome): Causes, Symptoms, and Treatment
Accutane Embryopathy (Fetal Retinoid Syndrome): Causes, Symptoms, and Treatment
Accutane (Isotretinoin) and Birth Defects: Latest Research and Safety Guidelines
Introduction
Accutane Embryopathy, also known as Fetal Retinoid Syndrome, is a rare but serious condition caused by maternal use of retinoids, specifically isotretinoin (Accutane), during pregnancy. Retinoids are synthetic derivatives of vitamin A commonly prescribed for severe nodular acne. Prenatal exposure can lead to a spectrum of physical and mental birth defects, even during the earliest weeks of pregnancy, often before a woman realizes she is pregnant.
Causes
The primary cause of Accutane embryopathy is maternal intake of isotretinoin or other retinoids during the first trimester. Other drugs in this class include:
- Actiretin (Soriatane)
- Etretinate (Tegison)
- Retinoin (Vesanoid)
Retinoids interfere with embryonic development, particularly in craniofacial structures, the heart, and the central nervous system. Even low doses may pose a risk, and birth defects can occur if exposure happens after the 15th day post-conception.
Risk Estimates:
- Medical literature suggests 25–35% risk of fetal retinoid syndrome after first-trimester exposure.
- Prospective studies indicate up to 42% of exposed infants may have abnormalities consistent with Accutane embryopathy.
Symptoms and Clinical Features
The presentation of Accutane embryopathy varies. Affected infants may have craniofacial, cardiac, CNS, and other systemic abnormalities.
Craniofacial Abnormalities
- Small, malformed, or missing ears (microtia or anotia)
- Cleft lip or palate
- Widely spaced eyes (hypertelorism)
- Small jaw (micrognathia) and flattened nasal bridge
- Facial nerve paralysis and abnormal hair patterns
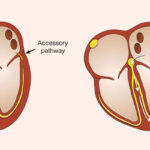
Cardiovascular Abnormalities
- Tetralogy of Fallot
- Ventricular septal defects (VSDs)
- Transposition of great arteries
- Hypoplastic aortic arch
- Double outlet right ventricle
Central Nervous System
- Microcephaly (small head)
- Hydrocephalus (fluid accumulation in the brain)
- Cerebellar hypoplasia
- Developmental delays and mild-to-moderate intellectual disability
- Holoprosencephaly in severe cases
Other Features
- Thymic aplasia or hypoplasia (affecting immunity)
- Syndactyly (webbed fingers)
- Skeletal malformations
- Low muscle tone (hypotonia)
Diagnosis
Diagnosis is primarily clinical, based on maternal history of isotretinoin use and recognition of characteristic birth defects.
- Prenatal Ultrasound may detect heart defects, hydrocephalus, cleft palate, or craniofacial abnormalities.
- Physical examination of the newborn reveals distinctive features.
- Genetic testing may rule out other syndromes.
Prognosis
- Prognosis depends on severity of abnormalities.
- Most individuals have a normal lifespan, but complex cardiac or CNS defects may be life-threatening.
- Cognitive impairment is common, even in the absence of major physical malformations.
Treatment
Management is primarily supportive and symptomatic:
- Cardiac surgery for congenital heart defects
- Shunt placement for hydrocephalus
- Speech therapy, behavioral therapy, and developmental support
- Multidisciplinary care involving cardiologists, neurologists, and craniofacial specialists
No cure exists for the syndrome itself; prevention is critical.
Prevention and Guidelines
Because isotretinoin is teratogenic (FDA Category X), strict pregnancy prevention measures are mandatory:
- Two negative pregnancy tests before starting therapy
- Two forms of contraception (hormonal + barrier) starting one month before, during, and one month after treatment
- Monthly pregnancy testing during therapy
- Patient education and informed consent before prescription
- 30-day prescription dispensing to reinforce monthly pregnancy testing
Isotretinoin should never be shared or purchased online without proper medical supervision.
Epidemiology
- Equal prevalence in males and females (based on exposure)
- Underdiagnosed due to unrecognized or unreported cases
- Most prescriptions are given to women under 30, reflecting the high-risk population
Historical Data:
- From 1982–2000, 1,995 exposed pregnancies were reported.
- 42% of delivered infants exhibited features of Accutane embryopathy.
Key Takeaways
- Accutane embryopathy is a preventable birth defect caused by isotretinoin exposure during early pregnancy.
- The most affected systems are craniofacial, cardiac, and central nervous system.
- Prevention via strict contraception, pregnancy testing, and patient education is the most effective strategy.
- Early diagnosis and multidisciplinary care improve outcomes for affected infants.
For Detailed Guides and Health Articles Visit: https://healthcaretipshub.com/